For some reason it just really bugs me that these two terms are incorrectly interchanged so frequently.
Part of the problem is that the document General Principles of Software Validation; Final Guidance for Industry and FDA Staff (2002) does not do a good job of differentiating actual verification and validation activities. They just call everything validation.
The recent MD&DI article Building Quality into Medical Device Software provides a pretty good overview of the these regulatory requirements, but is a another case in point. The article talks about "software validation" at every step just like the FDA document.
Another similar article on this subject is Software Validation: Turning Concepts into Business Benefits. It is also confused. e.g. (my highlight):
... software validation involves the execution of tests designed to cover each of the specific system requirements.
No, testing specific requirements is a verification activity! It's no wonder most people are confused.
These definitions, Difference between Verification and Validation, are better as they highlight the sequencing of activities:
Verification takes place before validation, and not vice versa. Verification evaluates documents, plans, code, requirements, and specifications. Validation, on the other hand, evaluates the product itself.
From here (warning PDF):
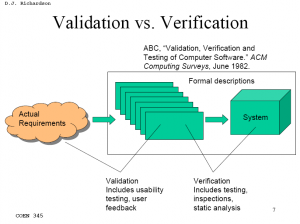
Validation activities (usability testing, user feedback, etc.) are much harder to define, execute, and document properly than most verification testing.
Here are the golden rules:
Verification: was the product built right?
Validation: was the right product built?
I guess I should get over it...
UPDATE (5/12/09): Good definitions from here: Diagnosing Medical Device Software Defects Using Static Analysis:
Verification and validation are terms that are often used in software. However, it is important to understand the difference between these two distinct but complementary activities. Software verification provides objective evidence that the design outputs of a particular phase of the software development life cycle meet all of the specified requirements for that phase by checking for consistency, completeness, and correctness of the software and its supporting documentation. Validation, on the other hand, is the confirmation by examination and provision of objective evidence that software specifications conform to user needs and intended uses, and that the particular requirements implemented through software can be consistently fulfilled.
UPDATE (8/6/09): The importance of proper V&V can not be overstated. The FDA is watching: FDA still enforcing regulations for validation of enterprise software.
UPDATE (2/11/10): I just noticed that the guidance document link on the FDA site was changed and fixed it. When I reviewed the document I found that even though it was "issued" in Jan. 2002 it had been recently updated (11/6/09). The later sections (4, 5, and 6) still use the term validation generically, but the updated document does distinguish between verification and validation:
3.1.2 Verification and Validation
The Quality System regulation is harmonized with ISO 8402:1994, which treats "verification" and "validation" as separate and distinct terms. On the other hand, many software engineering journal articles and textbooks use the terms "verification" and "validation" interchangeably, or in some cases refer to software "verification, validation, and testing (VV&T)" as if it is a single concept, with no distinction among the three terms.
Software verification provides objective evidence that the design outputs of a particular phase of the software development life cycle meet all of the specified requirements for that phase. Software verification looks for consistency, completeness, and correctness of the software and its supporting documentation, as it is being developed, and provides support for a subsequent conclusion that software is validated. Software testing is one of many verification activities intended to confirm that software development output meets its input requirements. Other verification activities include various static and dynamic analyses, code and document inspections, walkthroughs, and other techniques.
Software validation is a part of the design validation for a finished device, but is not separately defined in the Quality System regulation. For purposes of this guidance, FDA considers software validation to be "confirmation by examination and provision of objective evidence that software specifications conform to user needs and intended uses, and that the particular requirements implemented through software can be consistently fulfilled." In practice, software validation activities may occur both during, as well as at the end of the software development life cycle to ensure that all requirements have been fulfilled.
